“Bringing the right antigen to the right activated cell”
Pr. Yves Lévy, Chief Medical & Scientific Officer, Founder

LinKinVax’s platform is the work of the Vaccine Research Institute (VRI), created by Pr. Yves Lévy in 2011.
For ten years, the VRI, under the umbrella of INSERM and University Paris Est Créteil, has been leading 40 projects involving 150 researchers from 20 academic and industrial partners building a transdisciplinary approach from the basic sciences in the field of immunological, virological and translational research into the development of a clinical therapeutic and prophylactic program.
In the last years, the discovery path of the VRI was focused on the development of monoclonal antibodies targeting dendritic cells to educate and harness the immune system. Dendritic cells activate our adaptive immune system by presenting antigen to the immune system (B and T lymphocytes).
It resulted in a major leap forward: a dendritic cell-targeting immunotherapy platform using a safe and easy technology based upon monoclonal antibody fused to proteins matching antigens of a specific pathogens or cancer cells to trigger a strong and lasting immune response.
UNIQUE FEATURES OF LINKINVAX VERSATILE IMMUNOTHERAPY PLATFORM
Specific
Highly specific DC and pathogenic antigens targeting mAbs
Safe
Platform’s safety already confirmed in human (200+ injections in 70+ patients and healthy volunteers, with up to 2-years of follow-up)
Long-lasting
Re-programming of the immune system, subsequent immunological memory and long-term immunosurveillance
Universal
Commutable pathogenic antigens allows us to design our mAb for a broad spectrum of therapeutic applications
Easy-to-produce
Easy manufacturing and cost-effective
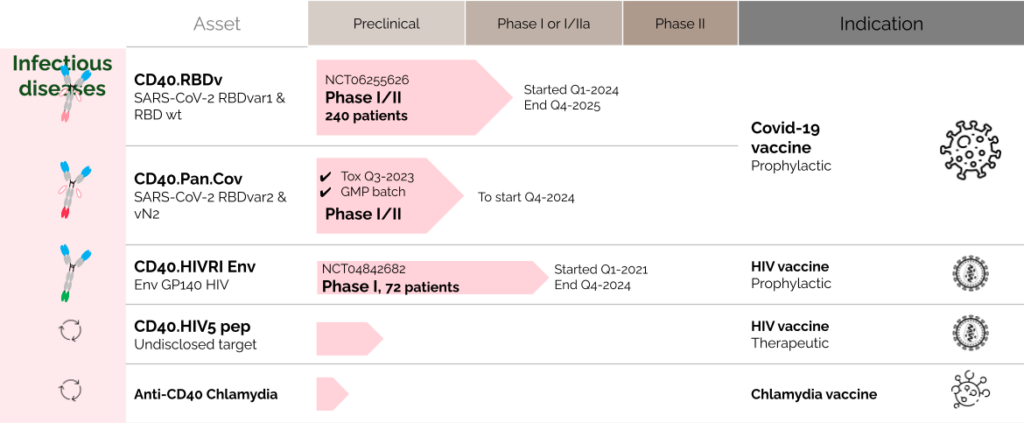
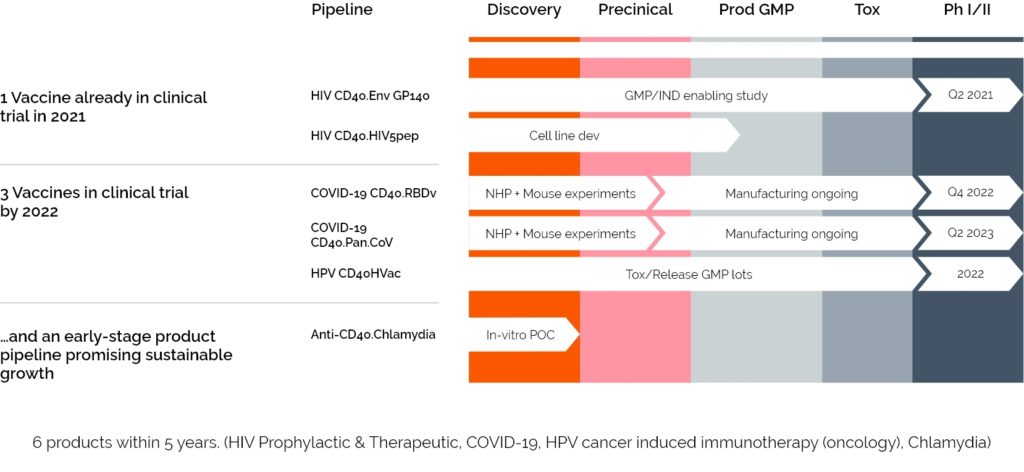
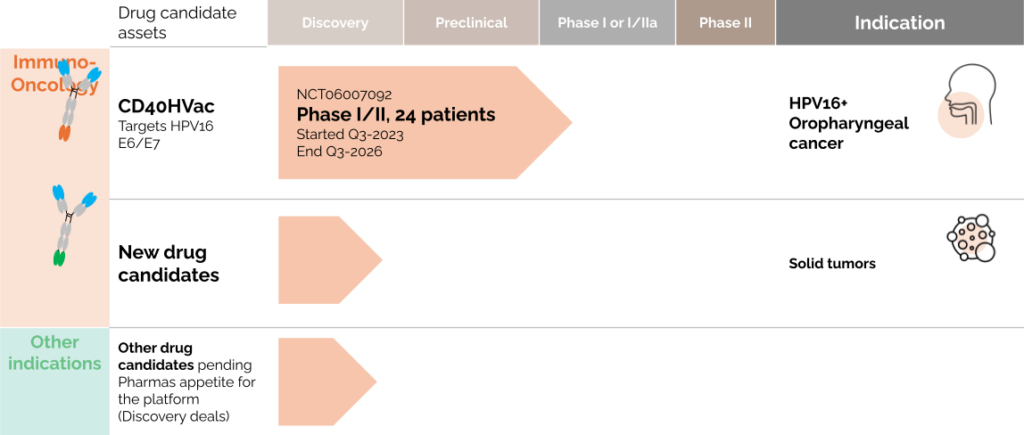
LinKinVax’s promising immunotherapy platform is already starting to deliver its first results with three candidates currently in clinical trials.
A PROPHYLACTIC HIV IMMUNOTHERAPY, ENTERED PHASE 1 CLINICAL TRIAL IN Q1 2021
A SARS-COV-2 IMMUNOTHERAPY,
ADDRESSING MULTI-EPITOPES AND VARIANTS, ENTERED PHASE 1 CLINICAL TRIAL IN Q1 2024
AN HPV induced CANCER IMMUNOTHERAPY ENTERED A PHASE 1 CLINICAL TRIAL IN Q3 2023
Today, LinKinVax has a diverse pipeline of immunotherapies targeting cancers and infectious diseases. LinKinVax’s pipeline is linked to the Vaccine Research Institute (VRI) development roadmap and is supporting advanced R&D to apply its platform to new pathogens and cancers.
A FIRST-CLASS immuno-oncology pipeline
oUR infectious diseases pipeline