“Bringing the right antigen to the right activated cell”
Pr. Yves Lévy, Chief Medical & Scientific Officer, Founder

LinKinVax’s universal vaccines platform is the work of the Vaccine Research Institute (VRI), created by Pr. Yves Lévy in 2011.
For ten years, the VRI, under the umbrella of INSERM and University Paris Est Créteil, has been leading 40 projects involving 150 researchers from 20 academic and industrial partners building a transdisciplinary approach from the basic sciences in the field of immunological, virological and translational research into the development of a clinical therapeutic and prophylactic program.
It resulted in a major leap forward in vaccine technology: a dendritic cell-targeting vaccine universal platform.
Dendritic cells activate our adaptive immune system by presenting antigen to B and T lymphocytes.
LinKinVax’s platform uses 30 years of protein vaccine experience to specifically target dendritic cells using a safe and easy technology to produce monoclonal antibody.
This monoclonal antibody is completed with proteins matching the antigen of a specific pathogen to trigger a strong and lasting immune response.
LinKinVax’s vaccines platform is efficient to develop prophylactic and therapeutic vaccines, adapt to new or fast mutating pathogens, enhance first generation vaccine, and use multiple booster doses.
LinKinVax’s promising vaccines platform is already starting to deliver its first results with three vaccines entering clinical trials in 2022/2023.

A prophylactic HIV vaccine, allowed to enter phase 1 clinical trial in S1 2021

A SARS-CoV-2 vaccine,
addressing multi-epitopes and variants, qualifying for clinical trial following very satisfying results in pre-clinical trial and planned to move to clinic in 2022
An HPV linked cancer vaccine allowed to enter phase 1 and 2 in 2022/2023
Today, LinKinVax has a particularly complete pipeline with 5 vaccines in market access over the next 5 years, including 3 in clinical trials in 2022 . LinKinVax’s pipeline is linked to the Vaccine Research Institute development roadmap and is supporting advanced R&D to apply its platform to new pathogens.
6 VACCINES AT VARIOUS STAGES OF CLINICAL DEVELOPMENT
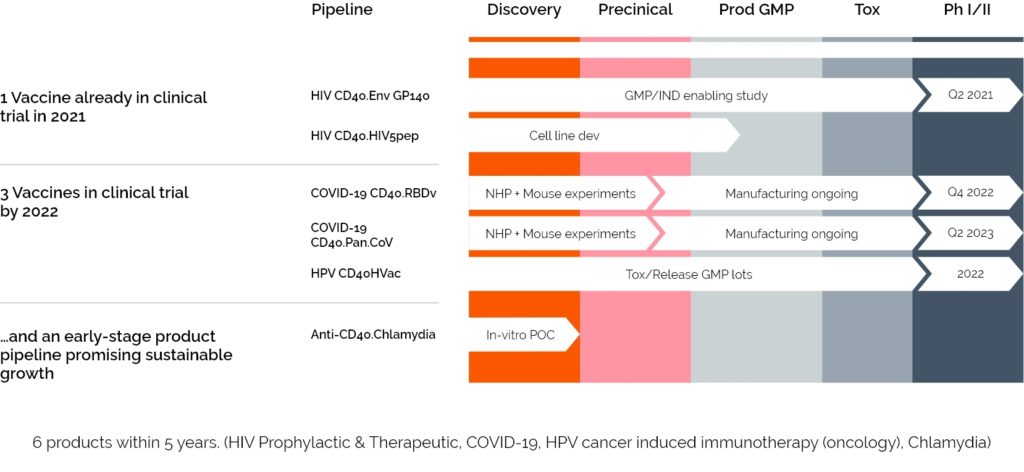
Market access offering on-shelf availability for 5 products within 5 years… (HIV Prophylactic & Therapeutic, COVID 19, HPV, Chlamydia)… and an early-stage product pipeline promising sustainable growth.
